AE, adverse event; BID, twice daily; BTK, Bruton’s tyrosine kinase; CI, confidence interval; CLL, chronic lymphocytic leukaemia; CR, complete response; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ITT, intention-to-treat; iwCLL, international workshop on CLL; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; PR-L, partial response with lymphocytosis; QD, once daily; R, randomisation; R/R, relapsed/refractory; SLL, small lymphocytic lymphoma.
References:
- BRUKINSA. European Union Summary of Product Characteristics. BeOne Medicines Ireland Limited;
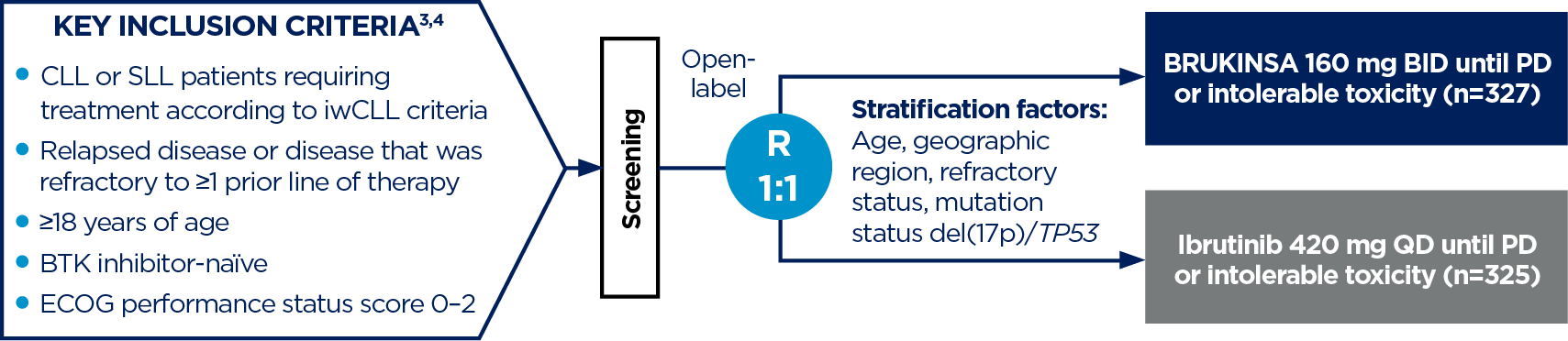
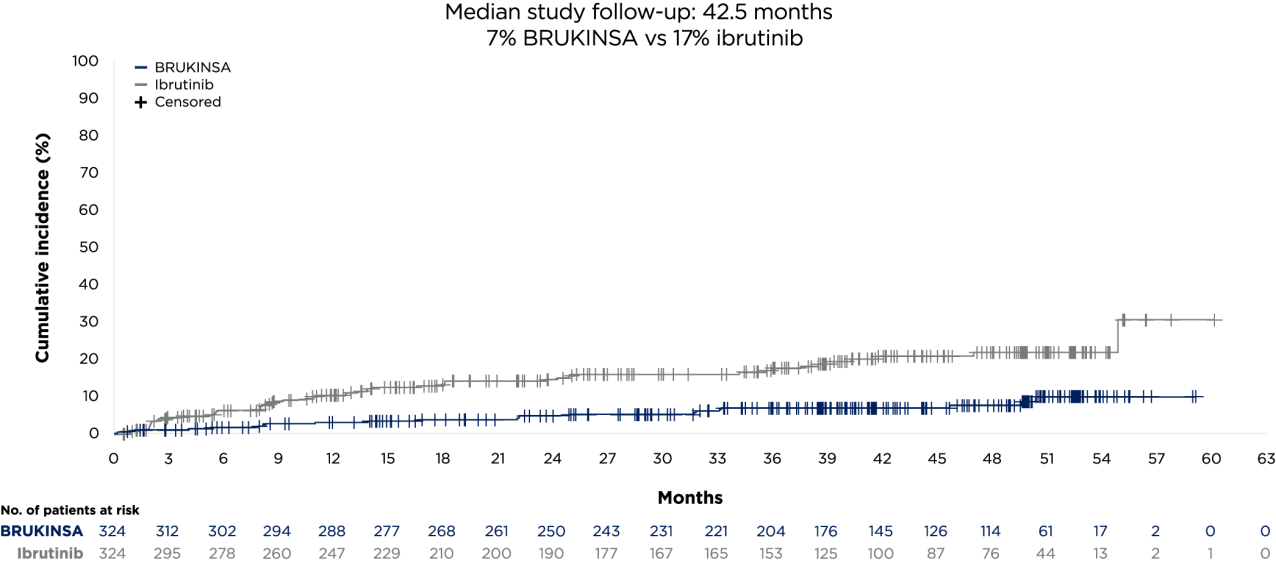
- Tam CS, et al. Blood Cancer J. 2023;13(1):141;
- Brown JR, et al. N Engl J Med. 2023;26;388(4):319–332;
- Hillmen P, et al. J Clin Oncol. 2023;41(5):1035–1045;
- Brown JR, et al. Blood. 2024;144(26):2706–2717;
- Byrd JC, et al. J Clin Oncol. 2021;39(31):3441–3452;
- Tam CS, et al. Expert Rev Clin Pharmacol. 2021;14(11):1329–1344;
- Brullo C, et al. Int J Mol Sci. 2021;22(14):7641;
- Shadman M, et al. Lancet Haematol. 2023;10(1):e35–e45;
- Guo Y, et al. J Med Chem. 2019;62(17):7923–7940;
- Imbruvica. Summary of Product Characteristics. Pharmacyclics LLC, Janssen Biotech, Inc;
- Calquence. Summary of Product Characteristics. AstraZeneca Pharmaceuticals LP.
The study is registered with ClinicalTrials.gov: NCT03734016
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Adverse events should be reported. Ireland: Healthcare professionals are asked to report any suspected adverse reactions via HPRA found at www.hpra.ie. Adverse events should also be reported to BeOne Medicines at adverse_events@beonemed.com
BRUKINSA and BeOne Medicines are trademarks owned by BeOne Medicines | GmbH or its affiliates. © BeOne Medicines | GmbH, 2025 All Rights Reserved.