BRUKINSA monotherapy is approved by HSE for WM in adults who have received at least one prior therapy, or as first-line treatment for patients unsuitable for chemo-immunotherapy.3
ASPEN was the first and only head-to-head BTKi trial in WM, with up to 6 years follow-up4–9
Waldenström’s macroglobulinaemia
Primary endpoint: CR+VGPR rate with BRUKINSA
vs ibrutinib in patients with MYD88 mutation4
28%
BRUKINSA
(n=29/102)
VS
19%
ibrutinib
(n=19/99)
There were no CRs in either treatment arm4
While the primary endpoint of superiority did not reach
statistical significance (p=0.09), numerically higher VGPR rates
were achieved in the BRUKINSA treatment arm at a median
follow-up of 19.4 months4
Numerically higher VGPR and MRR rates vs ibrutinib
at extended follow-up5
Median follow-up 44.4 months; patients with MYD88 mutation
VGPR
36%
BRUKINSA
(n=37/102)
VS
25%
ibrutinib
(n=25/99)
MRR
81%
BRUKINSA
VS
80%
ibrutinib
Median follow-up: 44.4 months; patients with MYD88 mutation
VGPR/CR in TN patients
37%
BRUKINSA
(n=7/19)
VS
22%
ibrutinib
(n=4/18)
VGPR/CR in patients with 1–3 lines prior therapy
37%
BRUKINSA
(n=28/76)
VS
26%
ibrutinib
(n=19/74)
Secondary endpoint: Responses in patients with
MYD88 wild type5
Investigator-assessed; median follow-up: 42.9 months
Adapted from Dimopoulos, et al. 2023.5
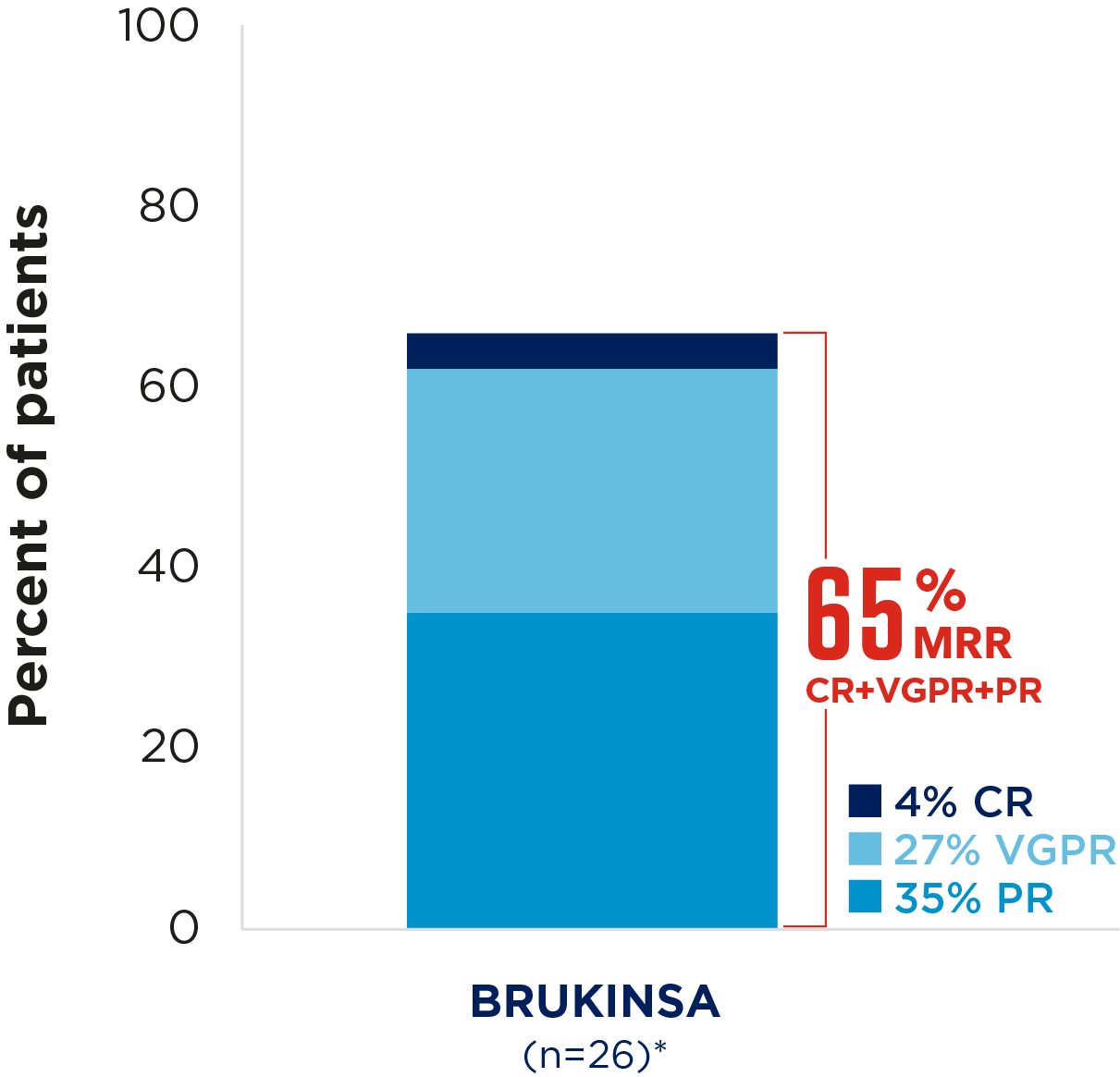
Best overall response rates in patients with WM receiving BRUKINSA9
Cohort 1: patients with MYD88 mutation, n=102;
Cohort 2: patients with MYD88 wild type, n=28; median follow-up 69.8 months

Adapted from D'Sa, et al. 2024.9

Significantly fewer GI symptoms were reported at the start of treatment with BRUKINSA than with ibrutinib
Cycle 4; (N=201)
Diarrhoea: p=0.01
Nausea/vomiting: p=0.01
Among patients achieving a VGPR, patient-reported
outcomes at 6 months showed a greater improvement with BRUKINSA than with ibrutinib
Cycle 25; (n=48)
Fatigue: p=0.0220
Physical functioning: p=0.0476
Any-grade; patients with >36 months follow-up
4%
BRUKINSA
(n=72)
VS
17%
ibrutinib
(n=64)
Treatment discontinuations due to AEs
9%
BRUKINSA
(n=9/101)
VS
20%
ibrutinib
(n=20/98)
Dose reductions due to AEs
16%
BRUKINSA
(n=16/101)
VS
27%
ibrutinib
(n=26/98)

