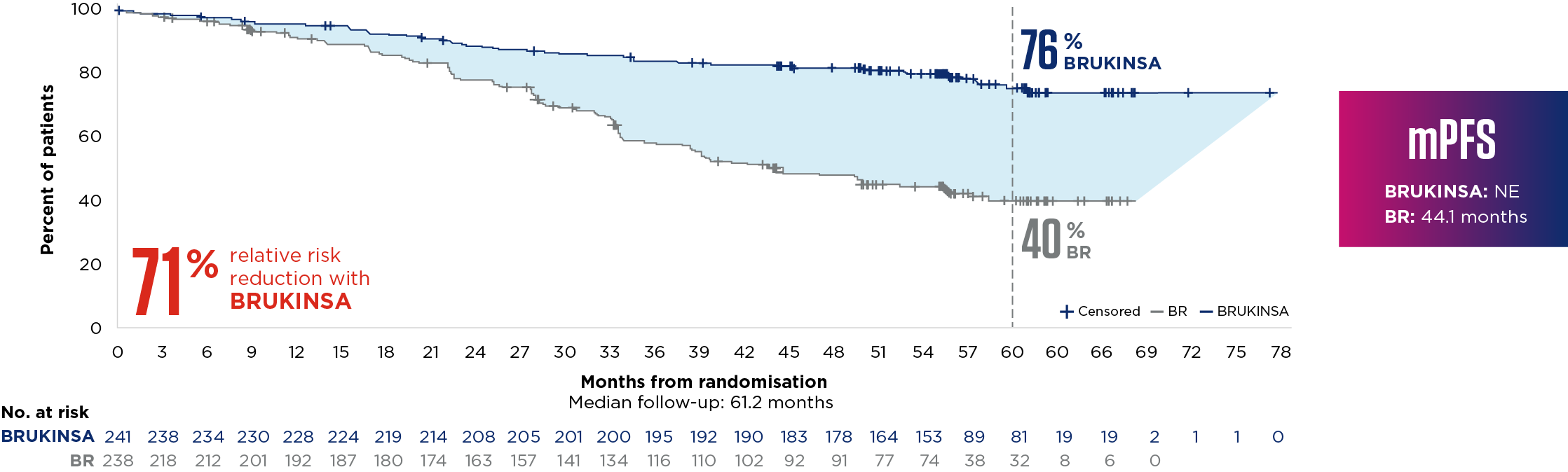
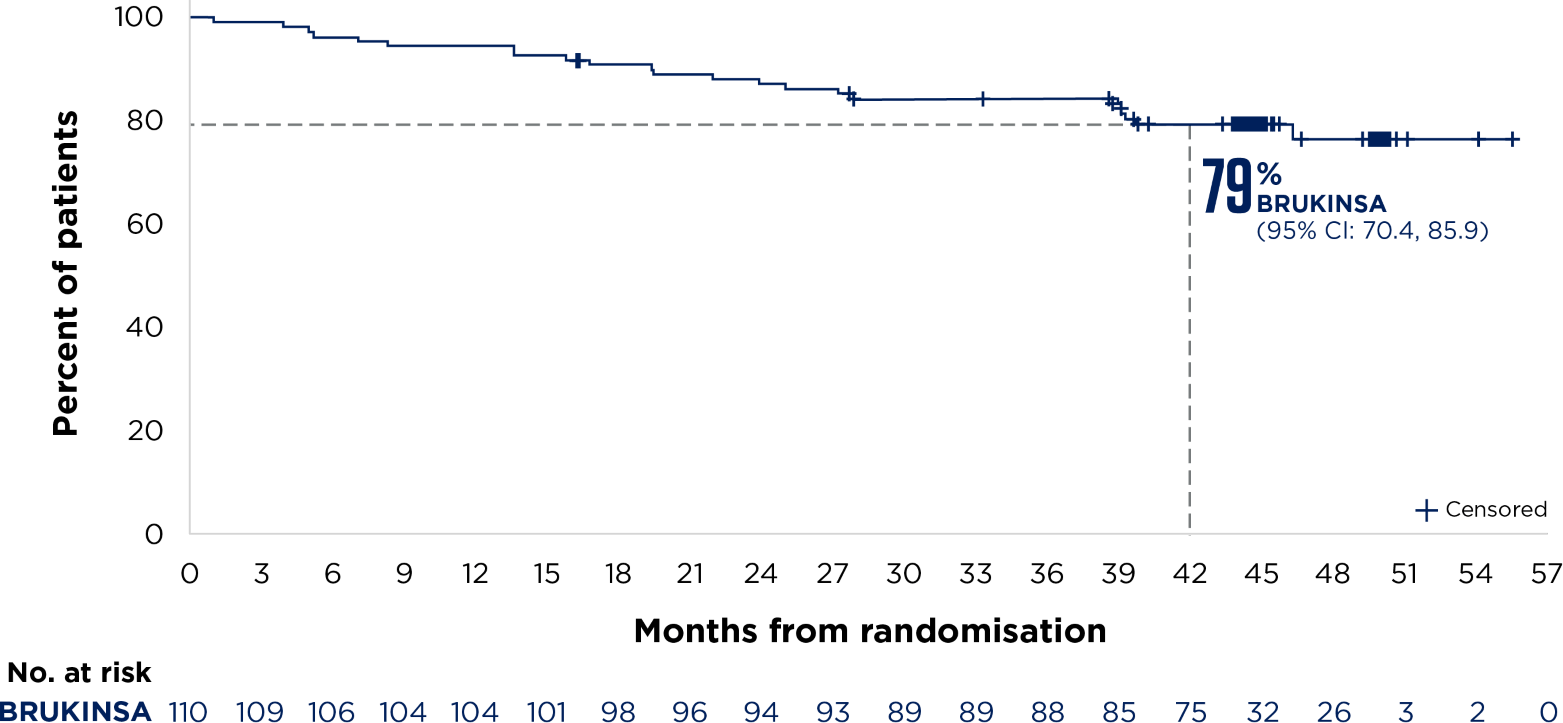
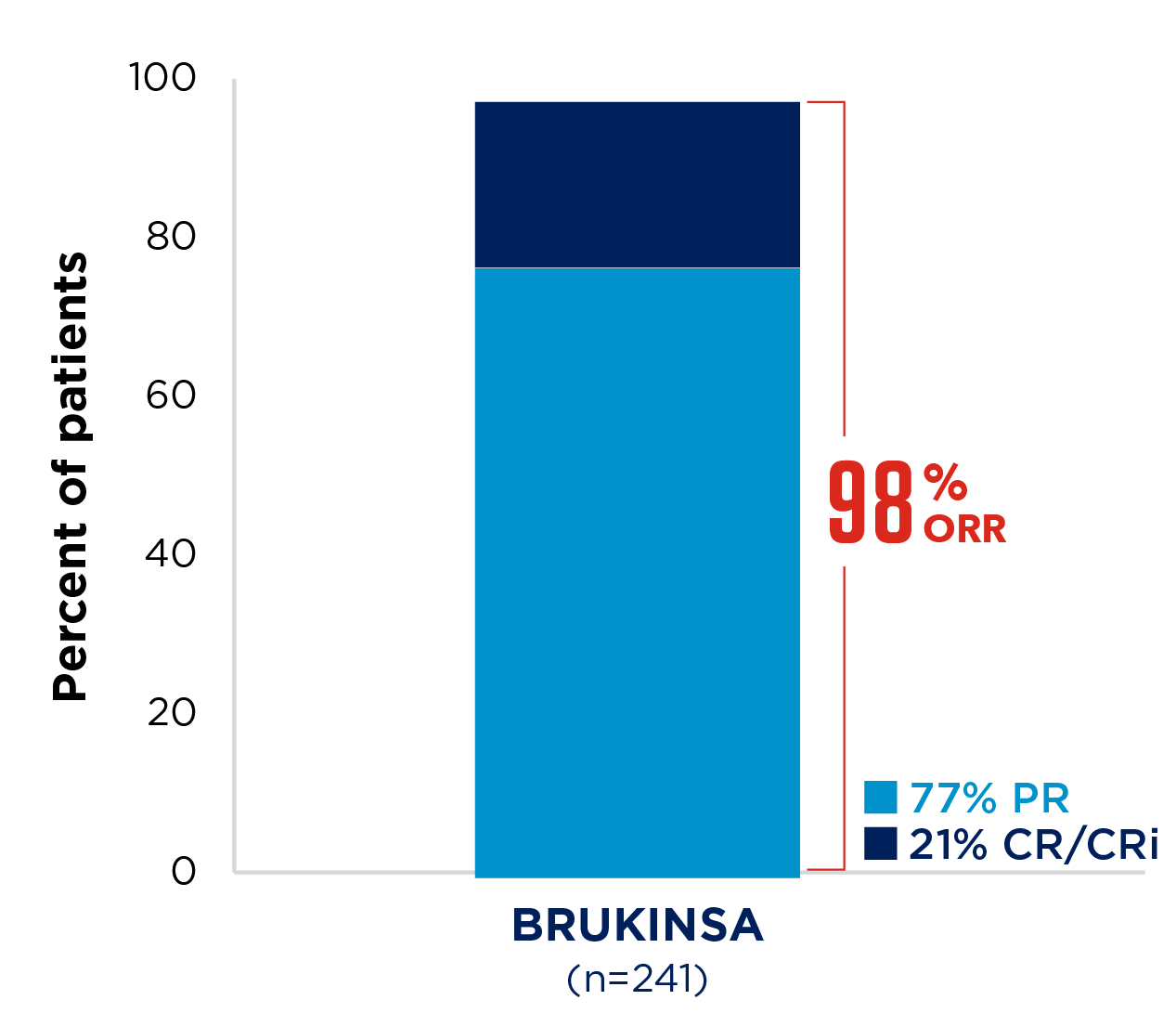
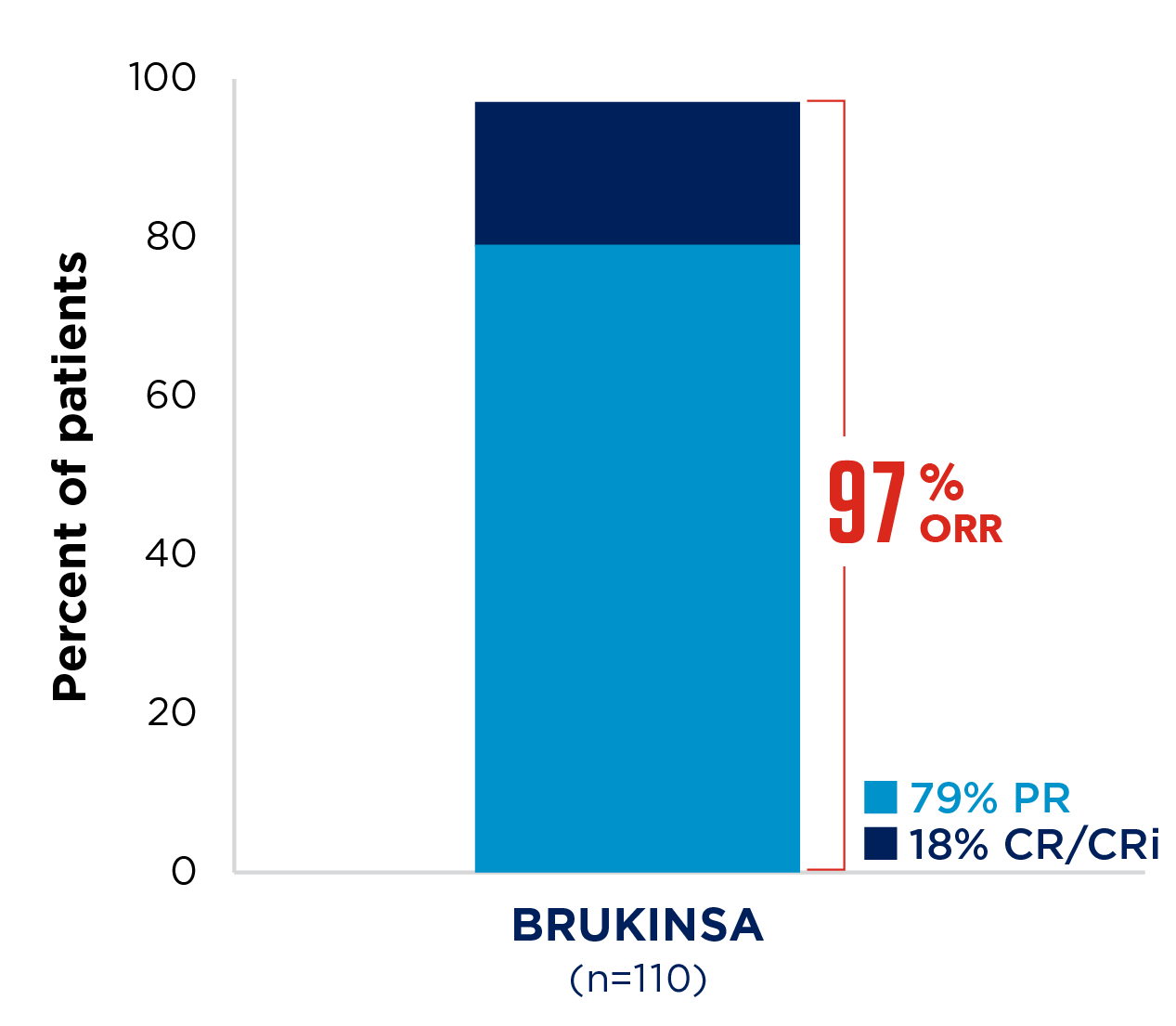
SEQUOIA was a randomised, open-label, Phase 3 trial of BRUKINSA vs BR in patients with TN CLL. Because patients with CLL/SLL whose tumours exhibit del(17p) have an unfavourable prognosis and respond poorly to standard CIT, patients with del(17p) mutations were assigned to receive BRUKINSA in a separate single-arm exploratory analysis. Cohort 1 enrolled patients without del(17p): BRUKINSA vs BR (n=479). Cohort 2 enrolled patients with del(17p); BRUKINSA single arm (n=111).4
ALPINE was an open-label, randomised, Phase 3, multicentre trial in patients with R/R CLL who received ≥1 prior systemic therapy.6
*The ELEVATE-RR study demonstrated noninferior PFS with acalabrutinib vs ibrutinib.7
AE, adverse event; BR, bendamustine + rituximab; BTK, Bruton’s tyrosine kinase; CI, confidence interval; CIT, chemo-immunotherapy; CLL, chronic lymphocytic leukaemia; CR, complete response including complete response with incomplete bone marrow recovery; GHS, global health status; GI, gastrointestinal; HSE, Health Service Executive; HR, hazard ratio; IGHV, immunoglobulin heavy chain variable region; IRC, independent review committee; mPFS, median progression-free survival; MZL, marginal zone lymphoma; NE, not estimable; ORR, overall response rate; PFS, progression-free survival; PR, partial response including nodular partial response; PR-L, partial response with lymphocytosis; QoL, quality of life; R/R, relapsed or refractory; SLL, small lymphocytic lymphoma; TN, treatment-naïve; WM, Waldenström’s macroglobulinaemia.
References:
- BRUKINSA. European Union Summary of Product Characteristics. BeOne Medicines Ireland Limited;
- Tam CS, et al. Blood Cancer J. 2023;13(1):141;
- HSE National Cancer Control Programme. Cancer Drugs Approved for Reimbursement. Available at: https://www.hse.ie/eng/services/list/5/cancer/profinfo/medonc/cdmp/new.html [Accessed December 2025];
- Tam CS, et al. Lancet Oncol. 2022;23(8):1031–1043;
- Hillmen P, et al. J Clin Oncol. 2023;41(5):1035–1045;
- Brown JR, et al. N Engl J Med. 2023;388(4):319–332;
- Byrd JC, et al. J Clin Oncol. 2021;39(31):3441–3452;
- Woyach JA. Am J Hematol. 2022;97(Suppl 2):S11–S18;
- Shadman M, et al. J Clin Oncol. 2025;43(7):780–787;
- Tam C, et al. ASCO Annual Meeting, May 30–June 3, 2025. Abstract 7011;
- Munir T, et al. EHA Congress, June 8–15, 2023. Abstract P639;
- Brown JR, et al. Blood. 2024;144(26):2706–2717;
- Tam CS, et al. Haematologica. 2021;106(9):2354–2363;
- Ghia P, et al. Curr Med Res Opin. 2023;39(11):1505–1511;
- Tam CS, et al. Expert Rev Clin Pharmacol. 2021;14(11):1329–1344;
- Brullo C, et al. Int J Mol Sci. 2021;22(14):7641;
- Shadman M, et al. Lancet Haematol. 2023;10(1):e35–e45;
- Guo Y, et al. J Med Chem. 2019;62(17):7923–7940;
- Imbruvica. Summary of Product Characteristics. Pharmacyclics LLC, Janssen Biotech, Inc.;
- Calquence. Summary of Product Characteristics. AstraZeneca Pharmaceuticals LP.
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Adverse events should be reported. Ireland: Healthcare professionals are asked to report any suspected adverse reactions via HPRA found at www.hpra.ie. Adverse events should also be reported to BeOne Medicines at adverse_events@beonemed.com
BRUKINSA and BeOne Medicines are trademarks owned by BeOne Medicines | GmbH or its affiliates. © BeOne Medicines | GmbH, 2025 All Rights Reserved.